The Chemical Language of Plant Defense: Volatile Organic Compounds (VOCs) and Tritrophic Interactions
Main Article Content
Abstract
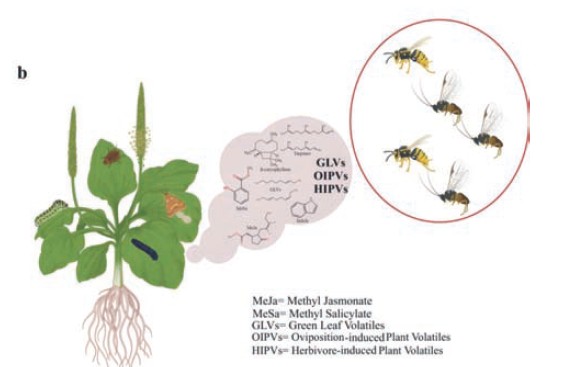
To defend themselves against herbivores, plants have evolved a sophisticated chemical "language" that mostly consists of volatile organic compounds (VOCs). By serving VOCs as signals to attract predators and parasitoids that prey on herbivores, these herbivore-induced plant volatiles (HIPVs) establish a tritrophic relationship that benefits plants and their ecological partners. In addition to discouraging herbivores, HIPVs are essential for communicating with neighbouring plants, which may "evesdrop" on these signals and bolster their defences. By interacting with higher trophic levels, this complex system highlights the deep ecological linkages that plants use to deal with challenges. Molecular processes including the jasmonic acid signalling system, which synchronizes plant defences against herbivory, control the synthesis of HIPVs. In addition to their ecological significance, HIPVs have a lot of promise for sustainable agriculture as they can replace conventional synthetic pesticides. These natural volatiles may boost biodiversity and increase crop resilience through integrated pest management (IPM). This study offers a thorough understanding of how plants use their chemical defences to survive and thrive by exploring the mechanisms behind HIPV production, their ecological roles and potential agricultural uses. This review provides the basis for sustainable pest control strategies by exploring the "chemical language" of plant defence and emphasizing VOCs' critical role in supporting tritrophic interactions.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
References
Aartsma, Y., Bianchi, F.J.J.A., van der Werf, W., Poelman, E.H. & Dicke, M. (2017). Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol., 216: 1054–1063.
Agrawal, A.A. (2000). Mechanisms, ecological consequences and agricultural implications of tritrophic interactions. Curr. Opin. Plant Biol., 3: 329–335.
Ali, M.Y., Naseem, T., Holopainen, J.K., Liu, T., Zhang, J., & Zhang, F. (2023). Tritrophic interactions among arthropod natural enemies, herbivores and plants considering volatile blends at different scale levels. Cells, 12(2): 251.
Ament, K., Kant, M.R., Sabelis, M.W., Haring, M.A. & Schuurink, R.C. (2004). Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol., 135: 2025–2037.
Arimura, G., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W. & Takabayashi, J. (2000). Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature, 406: 512–515.
Becker, C., Desneux, N., Monticelli, L., Fernandez, X., Michel, T., & Lavoir, A.-V. (2015). Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. BioMed Res. Int., 2015: 1–18.
Blande, J.D., Holopainen, J.K. & Niinemets, Ü. (2014). Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant, Cell Environ., 37: 1892–1904.
Böttger, A., Vothknecht, U., Bolle, C. & Wolf, A. (2018). Plant secondary metabolites and their general function in plants. In: Lessons on Caffeine, Cannabis & Co. Learning Materials in Biosciences. Springer, Cham.
Buonomo, B., Giannino, F., Saussure, S. & Venturino, E. (2019). Effects of limited volatiles release by plants in tritrophic interactions. Math. Biosci. Eng., 16: 3331–3344.
Choh, Y. & Takabayashi, J. (2010). Predator avoidance by phytophagous mites is affected by the presence of herbivores in a neighboring patch. J. Chem. Ecol., 36: 614–619.
Constabel, C.P., Bergey, D.R. & Ryan, C.A. (1995). Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc. Nat. Acad. Sci., 92: 407–411.
Croteau, R., Kutchan, T.M. & Lewis, N.G. (2000) Natural products (Secondary metabolites). Biochem. Mol. Biol. Plants, 24: 1250-1319.
Das, A., Lee, S.-H., Hyun, T.K., Kim, S.-W., & Kim, J.-Y. (2013). Plant volatiles as method of communication. Plant Biotechnol. Rep., 7: 9–26.
Das, S., Koner, A., Mobarak, S.H. & Barik, A. (2021). Attraction of the biocontrol agent, Lema praeusta, towards two Commelinaceae weed volatiles. J. Appl. Entomol., 145: 869–889.
Davidson-Lowe, E. & Ali, J.G. (2021). Herbivore-induced plant volatiles mediate behavioral interactions between a leaf-chewing and a phloem-feeding herbivore. Basic Appl. Ecol., 53: 39–48.
Debnath, R., Bhattacharyya, B., Koner, A. & Barik, A. (2023). Semiochemicals from Trichosanthes anguina (Cucurbitaceae) plants influence behavior in Diaphania indica. Pest Manag. Sci., 79: 4295–4308.
Dicke, M. (1999). Are herbivore‐induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl., 91: 131–142.
Dicke, M. (2015). Herbivore-induced plant volatiles as a rich source of information for arthropod predators: Fundamental and applied aspects. J. Indian Ins. Sci., 95: 35–42.
Dicke, M. & Baldwin, I.T. (2010). The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help.’ Trends Plant Sci., 15: 167–175.
Dicke, M. & Sabelis, M.W. (1988). How plants obtain predatory mites as bodyguards. Neth. J. Zool., 38: 148–165.
Dicke, M., van Loon, J.J.A. & Soler, R. (2009). Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol., 5: 317–324.
Dudareva, N., Klempien, A., Muhlemann, J.K. & Kaplan, I. (2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol., 198: 16–32.
Foba, C. N., Shi, J., An, Q., Liu, L., Hu, X., Hegab, M.A.M.S., Liu, H., Zhao, P. & Wang, M. (2023). Volatile-mediated tritrophic defense and priming in neighboring maize against Ostrinia furnacalis and Mythimna separata. Pest Manag. Sci., 79: 105–113.
Gebreziher, G.H. (2018). Herbivore-induced plant volatiles (HIPVs) in tritrophic interactions consisting of diverse herbivore species: A review. Int. J. Entomol. Res., 28: 28–33.
Gols, R., Roosjen, M., Dijkman, H. & Dicke, M. (2003). Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J. Chem. Ecol., 29:2651–2666.
Gols, R., Veenemans, C., Potting, R.P.J., Smid, H.M., Dicke, M., Harvey, J.A. & Bukovinszky, T. (2012). Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim. Behav., 83: 1231–1242.
Grof-Tisza, P., Turlings, T.C.J., Bustos-Segura, C. & Benrey, B. (2024). Field evidence for the role of plant volatiles induced by caterpillar oral secretion in prey localization by predatory social wasps. Biol. Lett., 20: 20240384.
Hartmann, T. (1996). Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol. Exp. Appl., 80: 177–188.
He, J., Fandino, R.A., Halitschke, R., Luck, K., Köllner, T.G., Murdock, M.H., Ray, R., Gase, K., Knaden, M., Baldwin, I.T. & Schuman, M.C. (2019). An unbiased approach elucidates variation in (S)-(+)-linalool, a context-specific mediator of a tritrophic interaction in wild tobacco. Proc. Nat. Acad. Sci. USA., 116: 14651–14660.
Heil, M. (2008). Indirect defence via tritrophic interactions. New Phytol., 178: 41–61.
Heil, M. (2014). Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol., 204: 297–306.
Heil, M. & Bueno, S.J.C. (2007). Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Nat. Acad. Sci., 104: 5467–5472.
Heil, M. & Karban, R. (2010). Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol., 25: 137–144.
Huang, W., Siemann, E., Yang, X., Wheeler, G.S. & Ding, J. (2013). Facilitation and inhibition: changes in plant nitrogen and secondary metabolites mediate interactions between above-ground and below-ground herbivores. Proc. R. Soc. B. Biol. Sci., 280: 20131318.
Hussein, A.R. & A. El-Anssary, A. (2019). Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In: Herbal Medicine. Intech Open. https://doi.org/10.5772/intechopen.76139
Jaworski, C.C., Xiao, D., Xu, Q., Ramirez‐Romero, R., Guo, X., Wang, S. & Desneux, N. (2019). Varying the spatial arrangement of synthetic herbivore‐induced plant volatiles and companion plants to improve conservation biological control. J. Appl. Ecol., 56: 1176–1188.
Karmakar, A., Mitra, S. & Barik, A. (2018). Systemically released volatiles from Solena amplexicaulis plant leaves with color cues influencing attraction of a generalist insect herbivore. Int. J. Pest Manag., 64: 210–220.
Kessler, A. & Baldwin, I.T. (2002). Plant responses to insect herbivory: the emerging molecular analysis. Ann. Rev. Plant Biol., 53: 299–328.
Khurshid, A., Inayat, R., Basit, A., Mobarak, S.H. & Liu, T.-X. (2024). Volatile cis-jasmone affects the tritrophic interactions among the potato plants, the green peach aphid (Myzus persicae), and the parasitoid (Aphidius gifuensis). Crop Prot., 184: 106870. https://doi.org/10.1016/j.cropro.2024.106870
Koner, A., Das, S., Karmakar, A. & Barik, A. (2022). Attraction of the biocontrol agent, Galerucella placida towards volatile blends of two Polygonaceae weeds, Rumex dentatus and Polygonum glabrum. J. Chem. Ecol., 48: 165–178.
Kost, C. & Heil, M. (2006). Herbivore‐induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol., 94: 619–628.
Maffei, M.E., Mithöfer, A. & Boland, W. (2007). Before gene expression: early events in plant–insect interaction. Trends Plant Sci., 12: 310–316.
Malik, U., Karmakar, A. & Barik, A. (2016). Attraction of the potential biocontrol agent Galerucella placida (Coleoptera: Chrysomelidae) to the volatiles of Polygonum orientale (Polygonaceae) weed leaves. Chemoecology, 26: 45–58.
Mitra, P., Das, S., Debnath, R., Mobarak, S.H. & Barik, A. (2021). Identification of Lathyrus sativus plant volatiles causing behavioral preference of Aphis craccivora. Pest Manag. Sci., 77: 285–299.
Mitra, P., Mitra, S. & Barik, A. (2022). Attraction of Aphis craccivora Koch (Hemiptera: Aphididae) towards Lathyrus sativus L. flower volatiles. Int. J. Pest Manag. https://doi.org/10.1080/09670874.2022.2088879
Mitra, S., Karmakar, A., Das, S. & Barik, A. (2020). Attraction of the potential biocontrol agent Altica cyanea by volatile compounds of three species of Ludwigia weeds from rice fields. Entomol. Exp. Appl., 168: 91–104.
Mitra, S., Karmakar, A., Mukherjee, A. & Barik, A. (2017). The role of leaf volatiles of Ludwigia octovalvis (Jacq.) Raven in the attraction of Altica cyanea (Weber) (Coleoptera: Chrysomelidae). J. Chem. Ecol., 43: 679–692.
Mobarak, S. H., Koner, A., Debnath, R., & Barik, A. (2022). The role of green gram plant volatile blends in the behavior of Arctiid moth, Spilosoma obliqua. J. Chem. Ecol., 48: 802–816.
Noge, K. & Tamogami, S. (2018). Isovaleronitrile co-induced with its precursor, l-leucine, by herbivory in the common evening primrose stimulates foraging behavior of the predatory blue shield bug. Biosci. Biotechnol. Biochem., 82: 395–406.
Pagare, S., Bhatia, M., Tripathi, N., Pagare, S. & Bansal, Y.K. (2015). Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm., 9: 293–304.
Paré, P. W. & Tumlinson, J.H. (1999). Plant volatiles as a defense against insect herbivores. Plant Physiol., 121: 325–332.
Pérez-Hedo, M., Alonso-Valiente, M., Vacas, S., Gallego, C., Pons, C., Arbona, V., Rambla, J.L., Navarro-Llopis, V., Granell, A. & Urbaneja, A. (2021). Plant exposure to herbivore-induced plant volatiles: a sustainable approach through eliciting plant defenses. J. Pest Sci., 94: 1221–1235.
Ramadan, A., Muroi, A. & Arimura, G. (2011). Herbivore-induced maize volatiles serve as priming cues for resistance against post-attack by the specialist armyworm Mythimna separata. J. Plant Interact., 6: 155–158.
Rashid, Md. H.-O. & Chung, Y.R. (2017). Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci., 8: 275240.
Riahi, C., Urbaneja, A., Gallego, C. & Pérez-Hedo, M. (2023). Exploring the potential of plant volatiles to enhance pest management in sweet pepper plants. IOBC-WPRS Bulletin, 167: 154-158.
Runyon, J.B., Mescher, M.C. & De Moraes, C.M. (2010). Plant defenses against parasitic plants show similarities to those induced by herbivores and pathogens. Plant Signal. Behav., 5: 929–931.
Sabelis, M.W. & van de Baan, H.E. (1983). Location of distant spider mite colonies by Phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol. Exp. Appl., 33: 303–314.
Sarkar, N., Karmakar, A. & Barik, A. (2016). Volatiles of Solena amplexicaulis (Lam.) Gandhi leaves influencing attraction of two generalist insect herbivores. J. Chem. Ecol., 42: 1004–1015.
Schuman, M.C., Barthel, K. & Baldwin, I.T. (2012). Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. ELife, 1: e00007.
Stout, M.J., Workman, J. & Duffey, S.S. (1994). Differential induction of tomato foliar proteins by arthropod herbivores. J. Chem. Ecol., 20: 2575–2594.
Sun, G., Zhang, X., Liu, Y., Chai, L., Liu, D., Chen, Z. & Lü, S. (2024). Plant chemical defenses against insect herbivores—using the wild tobacco as a model. Phyton, 93: 641–659.
Takabayashi, J. & Dicke, M. (1996). Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci., 1: 109–113.
Tatemoto, S. & Shimoda, T. (2008). Olfactory responses of the predatory mites (Neoseiulus cucumeris) and insects (Orius strigicollis) to two different plant species infested with onion thrips (Thrips tabaci). J. Chem. Ecol., 34: 605–613.
Thirumurugan, D., Cholarajan, A., Raja, S.S.S. & Vijayakumar, R. (2018). An introductory chapter: Secondary metabolites. In: Secondary Metabolites - Sources and Applications. InTech.
Tiku A.R. (2021) Direct and indirect defence against insects. In: I.K. Singh, A. Singh (eds.), Plant-pest interactions: From molecular mechanisms to chemical ecology. Springer Nature Singapore.
Tiwari, R. & Rana, C.S. (2015). Plant secondary metabolites: a review. Int. J. Eng. Res. Gen. Sci., 3: 661–670.
Turlings, T.C.J. & Wäckers, F. (2004). Recruitment of predators and parasitoids by herbivore-injured plants. In: R. D. Cardé, J. G. Millar (eds.), Advances in Insect Chemical Ecology (pp. 21–75). Cambridge University Press.
Turlings, T.C.J., Tumlinson, J.H. & Lewis, W.J. (1990). Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science, 250: 1251–1253.
Turlings, T.C. & Tumlinson, J.H. (1992). Systemic release of chemical signals by herbivore-injured corn. Proc. Nat. Acad. Sci., 89: 8399–8402.
Turlings, T.C., Loughrin, J.H., McCall, P.J., Röse, U.S., Lewis, W.J. & Tumlinson, J.H. (1995). How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Nat. Acad. Sci., 92: 4169–4174.
van den Boom, C.E.M., van Beek, T.A. & Dicke, M. (2002). Attraction of Phytoseiulus persimilis (Acari: Phytoseiidae) towards volatiles from various Tetranychus urticae -infested plant species. Bull. Entomol. Res., 92: 539–546.
van Poecke, R.M.P. & Dicke, M. (2002). Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J. Exp. Bot., 53: 1793–1799.
van Poecke, R.M.P., Posthumus, M.A. & Dicke, M. (2001). Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J. Chem. Ecol., 27: 1911–1928.
von Mérey, G.E., Veyrat, N., D’Alessandro, M. & Turlings, T. C. J. (2013). Herbivore-induced maize leaf volatiles affect attraction and feeding behavior of Spodoptera littoralis caterpillars. Front. Plant Sci., 4: 47918.
War, A.R., Hussain, B. & Sharma, H. C. (2013). Induced resistance in groundnut by jasmonic acid and salicylic acid through alteration of trichome density and oviposition by Helicoverpa armigera (Lepidoptera: Noctuidae). AoB Plants, 5: plt053.
War, A.R., Paulraj, M.G., Ahmad, T., Buhroo, A.A., Hussain, B., Ignacimuthu, S. & Sharma, H.C. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav., 7: 1306–1320.
Wei, J., Yan, L., Ren, Q., Li, C., Ge, F. & Kang, L. (2013). Antagonism between herbivore-induced plant volatiles and trichomes affects tritrophic interactions. Plant Cell Environ., 36: 315–327.
Yasa, V., Suroshe, S.S. & Nebapure, S.M. (2024). Behavioral response of zigzag ladybird beetle Cheilomenes sexmaculata to the HIPVs induced by cotton aphid, Aphis gossypii. Arthropod Plant Interact., 18: 771–780.
Yu, H., Zhang, Y., Wyckhuys, K.A.G., Wu, K., Gao, X. & Guo, Y. (2010). Electrophysiological and behavioral response of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ. Entomol., 39: 600–609.