Metagenomic Analysis of Gut Microbiota of Purple Moorhen (Porphyrio poliocephalus) from the Wetlands of Kerala: Implications for Avian Ecology and Crop Pest Management
Main Article Content
Abstract
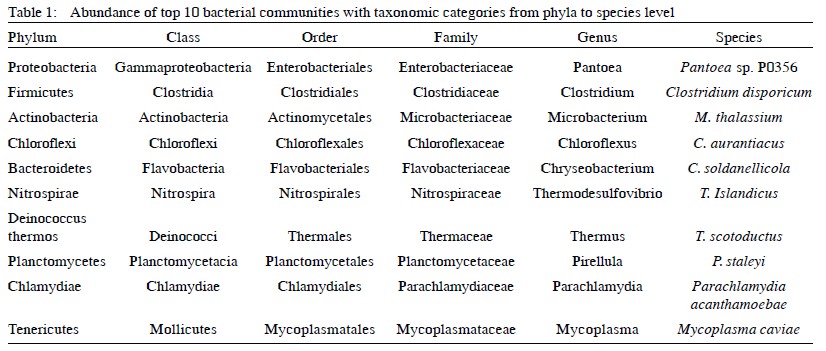
Wetlands are critical ecosystems known for their high biodiversity, supporting complex interactions among various species, including plants, birds, fish, and mollusks. They provide essential resources and ecosystem services that benefit local communities. The kole lands in Kerala serve as vital habitats for both, migratory and resident birds, including the Purple moorhen (Porphyrio poliocephalus), which is crucial for maintaining ecological balance. Understanding the gut microbiome of these birds is essential for insights into their health, ecology, and behavior. While substantial research subsists on mammalian and avian microbiomes remain less explored, especially in non-domesticated species like the purple Moorhen hence the present study exploits the metagenomic analysis of fecal pellets from Purple Moorhens to investigate the relationships between gut microbiota, avian health, and ecological roles. Fecal samples were collected, and metagenomic DNA was isolated and sequenced using next-generation Illumina sequencing. The analysis revealed a diverse bacterial community, with Proteobacteria as the dominant phylum followed by Firmicutes and Actinobacteria. The predominant bacterial species in the fecal pellet were Pantoea and Microbacterium thalassium, which play significant roles in digestion and pathogen resistance. The study helps understand the avian microbiome and suggests the potential eco-friendly pest management practices, leveraging the natural behaviors and microbiota of the Purple moorhen to support sustainable agriculture.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
References
Andreote, F. D., Jiménez, D. J., Chaves, D., Dias, A. C. F., Luvizotto, D. M., Dini-Andreote, F., Fasanella, C. C., Lopez, M. V., Baena, S., Taketani, R. G., & De Melo, I. S. (2012). The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS One, 7(6), e38600. https://doi.org/10.1371/journal.pone.0038600
Barbosa, A., Balagué, V., Valera, F., Martínez, A., Benzal, J., Motas, M., Diaz, J. I., Mira, A., and Pedrós-Alió, C. (2016). Age-related differences in the gastrointestinal microbiota of chinstrap penguins (Pygoscelis antarctica). PLOS ONE, 11(1), e0153215. https://doi.org/10.1371/journal.pone.0153215
Brisbin, J. T., Gong, J., & Sharif, S. (2008). Interactions between commensal bacteria and the gut-associated immune system of the chicken. Animal Health Research Reviews, 9(1), 101-110. https://doi.org/10.1017/S146625230800145X
Bryant, D. A., & Frigaard, N. U. (2006). Prokaryotic photosynthesis and phototrophy illuminated. Trends in Microbiology, 14(11), 488-496.
Charimba, G. (2012). The taxonomy and significance of Chryseobacterium isolates from poultry (Doctoral dissertation, University of the Free State).
Corsaro, D., & Greub, G. (2006). Pathogenic Potential of Novel Chlamydiae and Diagnostic Approaches to Infections Due to These Obligate Intracellular Bacteria. Clinical Microbiology Reviews, 19(2), 283-297. https://doi.org/10.1128/CMR.19.2.283-297.2006
Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior. Nature Reviews Neuroscience, 13(10), 701-712. https://doi.org/10.1038/nrn3346
Dewar, M. L., Arnould, J. P. Y., Dann, P., Trathan, P., Groscolas, R., & Smith, S. (2013). Interspecific variations in the gastrointestinal microbiota in penguins. MicrobiologyOpen, 2(1), 195-204. https://doi.org/10.1002/mbo3.57
Eriksson, P., Mourkas, E., González-Acuna, D., Olsen, B., & Ellström, P. (2017). Evaluation and optimization of microbial DNA extraction from fecal samples of wild Antarctic bird species. Infection Ecology and Epidemiology, 7, Article 1386536. https://doi.org/10.1080/20008686.2017.1386536
Gibbs, P. S., Kasa, R., Newbrey, J. L., Petermann, S. R., Wooley, R. E., Vinson, H. M., & Reed, W. (2007). Identification, antimicrobial resistance profiles, and virulence of members from the family Enterobacteriaceae from the feces of yellow-headed blackbirds (Xanthocephalus xanthocephalus) in North Dakota. Avian Diseases, 51(3), 649-655.
Godoy-Vitorino, F., Goldfarb, K. C., Brodie, E. L., Garcia-Amado, M. A., Michelangeli, F., and Dominguez-Bello, M. G. (2010). Developmental microbial ecology of the crop of the folivorous hoatzin (Opisthocomus hoazin). The ISME Journal, 4(5), 611-620. https://doi.org/10.1038/ismej.2010.9
Grond, K., Sandercock, B. K., Jumpponen, A., & Zeglin, L. H. (2018). The avian gut microbiota: community, physiology and function in wild birds. Journal of Avian Biology, 49, e01788. https://doi.org/10.1111/jav.01788
Hird, S. M. (2017). Evolutionary biology needs wild microbiomes. Frontiers in Microbiology, 8, 1-10. https://doi.org/10.3389/fmicb.2017.00725
Johnson, K. B., Stockwell, V. O., & Sawyer, T. L. (1993). Mechanisms of antagonism of the fire blight pathogen Erwinia amylovora by the bacterial biocontrol agents Pseudomonas fluorescens and Pantoea agglomerans. Phytopathology, 83(9), 982-990. https://doi.org/10.1094/Phyto-83-982
Johnson, K. B., & Stockwell, V. O. (1998). Biological control of fire blight: Understanding mechanisms and improving efficacy. Phytopathology, 88(6), 475-479. https://doi.org/10.1094/PHYTO.1998.88.6.475
Johnson, K. B., Temple, T. N., & Stockwell, V. O. (2000). Preliminary results on the interaction of biological agents with oxytetracycline in the management of fire blight. Acta Horticulturae, 551, 159-165.
Kohl, K. D. (2012). Diversity and function of the avian gut microbiota. Journal of Avian Biology, 43, 154-162. https://doi.org/10.1111/j.1600-048X.2012.05578.x
Mwanza, E. P., Hugo, A., Charimba, G., & Hugo, C. J. (2022). Pathogenic potential and control of Chryseobacterium species from clinical, fish, food, and environmental sources. Microorganisms, 10(5), 895. https://doi.org/10.3390/microorganisms10050895
Oakley, B. B., Lillehoj, H. S., Kogut, M. H., Kim, W. K., Maurer, J. J., Pedroso, A., Lee, M. D., Collett, S. R., Johnson, T. J., & Cox, N. A. (2014). The poultry gut microbiome and its relationship to health and disease. Frontiers in Veterinary Science, 1, 10. https://doi.org/10.3389/fvets.2014.00010
Pileggi, S. A. V., Pileggi, M., Olchanheski, L. R., Pileggi, M. E. V., & de Souza, M. M. (2012). Biodegradation of herbicides 2,4-D and picloram by Pantoea ananatis strain MMB-1. Brazilian Journal of Microbiology, 43(1), 177-183. https://doi.org/10.1590/S1517-83822012000100020
Regnaut, S., Lucas, F. S., & Fumagalli, L. (2006). DNA degradation in avian faecal samples and feasibility of non-invasive genetic studies of threatened capercaillie populations. Conservation Genetics, 7(3), 449-453. https://doi.org/10.1007/s10592-005-9046-6
Ruiz-Rodríguez, M., Lucas, S., Heeb, P., De Neve, L., Martı, J. G., & Soler, M. (2009a). Bacterial diversity at the cloaca relates to an immune response in magpie Pica pica and to the body condition of great spotted cuckoo Clamator glandarius nestlings. Journal of Avian Biology, 40(4), 452-460. https://doi.org/10.1111/j.1600-048X.2009.04800.x
Ruiz-Rodríguez, M., Lucas, S., Heeb, P., & Soler, J. J. (2009b). Body condition and immune response of great spotted cuckoo and magpie host nestlings. Journal of Avian Biology, 40(3), 306-311. https://doi.org/10.1111/j.1600-048X.2008.04623.x
Teyssier, A., Rouffaer, L. O., Saleh Hudin, N. S., Strubbe, D., Matthysen, E., Lens, L., & White, J. (2018). Inside the guts of the city: Urban-induced alterations of the gut microbiota in a wild passerine. Scientific Reports, 8(1), 11777. https://doi.org/10.1038/s41598-018-29988-9
Teyssier, A., Tolsma, S., Katsis, A. C., Holveck, M. J., & Dechaume-Moncharmont, F. X. (2018). Changes in gut microbiota do not correspond to variation in mating behavior in an avian mutualist. Microbial Ecology, 76(3), 709-720. https://doi.org/10.1007/s00248-018-1178-8
Torok, V. A., Allison, G. E., Percy, N. J., Ophel-Keller, K., & Hughes, R. J. (2011). Influence of antimicrobial feed additives on broiler commensal post hatch gut microbiota development and performance. Applied and Environmental Microbiology, 77(10), 3380-3390. https://doi.org/10.1128/AEM.02727-10
Trevelline, B. K., Fontaine, S. S., Hartup, B. K., & Kohl, K. D. (2019). Conservation biology needs a microbial renaissance: A call for the consideration of host-associated microbiota in wildlife management practices. Proceedings of the Royal Society B: Biological Sciences, 286, Article 20182448. https://doi.org/10.1098/rspb.2018.2448
Van Dongen, W. F., White, J., Brandl, H. B., Moodley, Y., Merkling, T., Leclaire, S., Blanchard, P., Danchin, É., Hatch, S. A., & Wagner, R. H. (2013). Age-related differences in the cloacal microbiota of a wild bird species. BMC Ecology, 13, 11. https://doi.org/10.1186/1472-6785-13-11
Videvall, E., Song, S. J., Bensch, H. M., Strandh, M., Engelbrecht, A., Serfontein, N., Hellgren, O., Olivier, A., Cloete, S., Knight, R., & Cornwallis, C. K. (2019). Major shifts in gut microbiota during development and its relationship to growth in ostriches. Molecular Ecology, 28(12), 2656-2672. https://doi.org/10.1111/mec.15087
Wilpiszeski, R. L., Zhang, Z., & House, C. H. (2018). Biogeography of thermophiles and predominance of Thermus scotoductus in domestic water heaters. Extremophiles, 23(1), 119-132. https://doi.org/10.1007/s00792-018-1066-z
Yang, C. M., Cao, G. T., Ferket, P. R., Liu, T. T., Zhou, L., Zhang, L., & Xiao, Y. P. (2012). Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poultry Science, 91(9), 2121-2129. https://doi.org/10.3382/ps.2012-02131